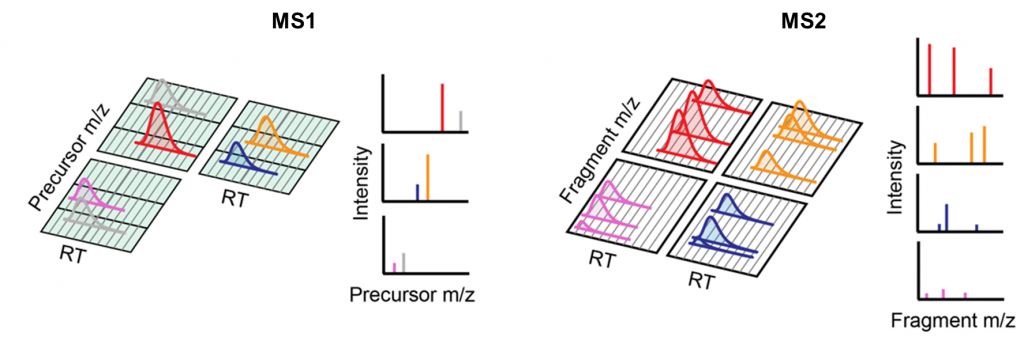
Narrower isolation windows in DIA increase the spectral specificity. However, since more windows are needed to cover the entire scan range, cycle time is lengthened leading to decreased overall analysis cycles. To balance window width with cycle time, an emerging development of DIA-MS uses gas-phase fractionation (GPF) to split the scan range into multiple fractions, each acquired at a different injection (Fig. 1). Several schemes have been successively developed to optimize the number of fractions, the widths and the overlaps of the isolation windows, the scan ranges, or the data processing strategies, including PAcIFIC, qPAcIFIC, aPAcIFIC, WiSIM-DIA, encyclopedia, PASS-DIA, pulseDIA, etc. (1-6). By combining the results from multiple fractions, the resulting proteome achieves a deeper coverage in identification and a broader dynamic range in quantification. For example, it has been shown that a five-fold fractionation using pulseDIA can increase the peptide identification number by 50% and the protein groups identification number by 29% than the windowed DIA schemes (6). Extreme cases include a 67-fold fractionation in PAcIFIC (1) and a 2 m/z isolation window in PASS-DIA (5).
Biological applications have been landed with EncyclopeDIA (7) and pulseDIA (8) during the past years. An enlengthened measurement timespan and increased sample consumption need to be taken into consideration when designing experiments.
Additionally, the whole story will be different if state-of-the-art mass spectrometers enabling a scanning rate of 200 to 300 Hz, i.e., Thermo Astral and Bruker timsTOF Ultra, are acquired. With more windows enabling a specificity comparable to DDA-MS, cycle time does not have to be compromised (9). Opportunities that “shake up the proteomics field” (10) and probably also the peptidome / lipidome field shall also arise, albeit it may take several years for the new-gen mass specs to be popularized among institutes and companies.
References
1. Panchaud A, Scherl A, Shaffer SA, von Haller PD, Kulasekara HD, Miller SI, et al. Precursor acquisition independent from ion count: how to dive deeper into the proteomics ocean. Anal Chem. 2009;81(15):6481-8.
2. Panchaud A, Jung S, Shaffer SA, Aitchison JD, Goodlett DR. Faster, quantitative, and accurate precursor acquisition independent from ion count. Analytical chemistry. 2011;83(6):2250-7.
3. Koopmans F, Ho JTC, Smit AB, Li KW. Comparative Analyses of Data Independent Acquisition Mass Spectrometric Approaches: DIA, WiSIM-DIA, and Untargeted DIA. PROTEOMICS. 2018;18(1):1700304.
4. Searle BC, Pino LK, Egertson JD, Ting YS, Lawrence RT, MacLean BX, et al. Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry. Nat Commun. 2018;9(1):5128.
5. Mun D-G, Renuse S, Saraswat M, Madugundu A, Udainiya S, Kim H, et al. PASS-DIA: A Data-Independent Acquisition Approach for Discovery Studies. Analytical Chemistry. 2020;92(21):14466-75.
6. Cai X, Ge W, Yi X, Sun R, Zhu J, Lu C, et al. PulseDIA: Data-Independent Acquisition Mass Spectrometry Using Multi-Injection Pulsed Gas-Phase Fractionation. Journal of Proteome Research. 2021;20(1):279-88.
7. Pino LK, Baeza J, Lauman R, Schilling B, Garcia BA. Improved SILAC Quantification with Data-Independent Acquisition to Investigate Bortezomib-Induced Protein Degradation. Journal of proteome research. 2021;20(4):1918-27.
8. Gao H, Zhang F, Liang S, Zhang Q, Lyu M, Qian L, et al. Accelerated Lysis and Proteolytic Digestion of Biopsy-Level Fresh-Frozen and FFPE Tissue Samples Using Pressure Cycling Technology. J Proteome Res. 2020;19(5):1982-90.
9. Guzman UH, Martinez-Val A, Ye Z, Damoc E, Arrey TN, Pashkova A, et al. Ultra-fast label-free quantification and comprehensive proteome coverage with narrow-window data-independent acquisition. Nature Biotechnology. 2024.
10. Kuster B, Tüshaus J, Bayer FP. A new mass analyzer shakes up the proteomics field. Nature Biotechnology. 2024.